How to DIY titanium anodizing (Phosphoric acid)
Speaking of titanium, it has a very interesting property that it can be made into a very bright color appearance by an oxide film.
There are many things that use this characteristic to make accessories and cups with titanium.
We will introduce how to attach such an oxide film of titanium cleanly at home.
This time, it is a method called anodizing that uses a chemical called phosphoric acid to flow electricity.
What is titanium anodizing?
As I mentioned at the beginning, titanium looks in various colors depending on the thickness of the oxide film.
This phenomenon is caused by the interference of light, but if the thickness of this oxide film is controlled, the color of the desire can be colored.
There are various ways to control this thickness, but a common method is anodizing that uses electricity.
When electricity is released, the thickness of the oxide film becomes a certain thickness depending on the voltage.
The principle is used, but there are few conditions such as the voltage on the net, and there are quite a few technical books.
I do not know what to do even if I want to do it myself, so I have no choice but to try various things myself, but I would like you to save time for people who are trying to do anodizing by themselves in the same way by publishing on a blog.
What to prepare
The following are necessary for anodizing titanium.
- rectifier
- solution
- anode
- cathode
- a container for which a solution is placed
There are various types of solutions in this, and this time I summarize the results of using phosphoric acid in this article.
There is also a way to use sulfuric acid, which is summarized in a separate article.
Everything other than the solution is common, and everything other than the solution is summarized in the article of sulfuric acid here, so if you are concerned about anything other than the solution, please see this article together.
Phosphate
Phosphoric acid can also be purchased online.
I bought the following:
I do not know the concentration of what can be purchased by online mail order well, but I think that most of them are notations such as “85% or more".
I tried searching variously on the net, but speaking of phosphoric acid, 85%? Because it was like, I wonder if there is no problem with this.
Methods of anodizing
It is how to actually work, but the detailed explanation is also summarized in the above-mentioned sulfuric acid edition, so please check that one.
Preparation of the solution
For the solution, it is necessary to adjust the concentration.
When using phosphoric acid, I tried various papers that can be googled how much concentration to use, but 10% to 20% seemed to be good, so this time I decided to adjust it to 10%.
The original concentration was 85% or more, but it is thought that it is 85%, and when calculated, it is good to dilute it 8.5 times.
I want to adjust 200 ml and put it in a beaker, so I took 23 ml of phosphoric acid and put tap water there so that it would be 200 ml.
preprocessing
Before actually running electricity, the surface of titanium is chemically cleaned so that the oxidation reaction proceeds smoothly.
This pretreatment has a process called etching and a process of smut removal.
If you want to produce a color of the blue system up to 40V, a beautiful color will come out without pretreatment, but it is essential especially if you want to put out the color of the high voltage part with pure titanium.
More information is summarized in this article. Please take a look at it.
Tested conditions
Under such conditions, I actually anodized titanium in the following voltage setting and time. The results are summarized in tables and photos.
Both use industrial pure titanium.
From 1 to 8, mirror gloss finishes are used, and 9 to 24 are repeatedly etched, and matte-like ones are used.
When applying a voltage with it, let’s raise the voltage with titanium attached to the solution.
It is not a problem at low voltage, but it seems that sparks are easy to come out because electricity flows at a stretch at high voltages. It is not that the spark is dangerous, but the color does not come out beautifully.
| No… | Voltage | Time | Results |
|---|---|---|---|
| 1 | 10V | 10 minutes | Slightly dark yellow |
| 2 | 16V | 5 mins | Dark purple. dark. Is the redness strong, too? |
| 3 | 20V | 10 minutes | Dark Blue Purple |
| 4 | 38V | 10 minutes | Blue |
| 5 | 60V | 10 minutes | yellow. whitish than one |
| 6 | 80V | 10 minutes | tea. It looks pinky. |
| 7 | 100V | 8 mins | Uses a matte-like ring. There was no bright color. |
| 8 | 114V | 10 minutes | Uses a matte-like ring. There was no bright color. |
| Nine. | 110V | About 30 seconds | Slightly grayish color. green |
| 10 | 105V | About 30 seconds | Slightly grayish color. green |
| 11 | 100V | About 30 seconds | green. Yellow is strong, it feels like lime green. |
| 12 | 95V | About 30 seconds | green. There is a slight bluness. |
| Thirteen. | 90V | About 30 seconds | green. The blue has increased. |
| Fourteen. | 85V | About 30 seconds | Blue |
| Fifteen. | 80V | About 30 seconds | Purple |
| Sixteen. | 75V | About 30 seconds | Reddish Purple |
| Yao-yeon | 70V | About 30 seconds | Reddish Purple |
| Eighteen. | 65V | About 30 seconds | Pinkish Purple |
| Nineteen. | 60V | About 30 seconds | pink. There is yellow, too. |
| Twenty. | 55V | About 30 seconds | Yellow |
| 21. | 50V | About 30 seconds | yellow. Slightly lighter in color |
| 22 | 45V | About 30 seconds | yellow? light blue? The color is a little light. |
| 23 | 40V | About 30 seconds | Blue |
| 24 | 35V | About 30 seconds | Blue |
Here is a photo of the color made by each condition. The first half uses titanium finished with a mirror gloss. At this time, I had no knowledge of pretreatment such as etching, so I flow electricity directly without any pretreatment.





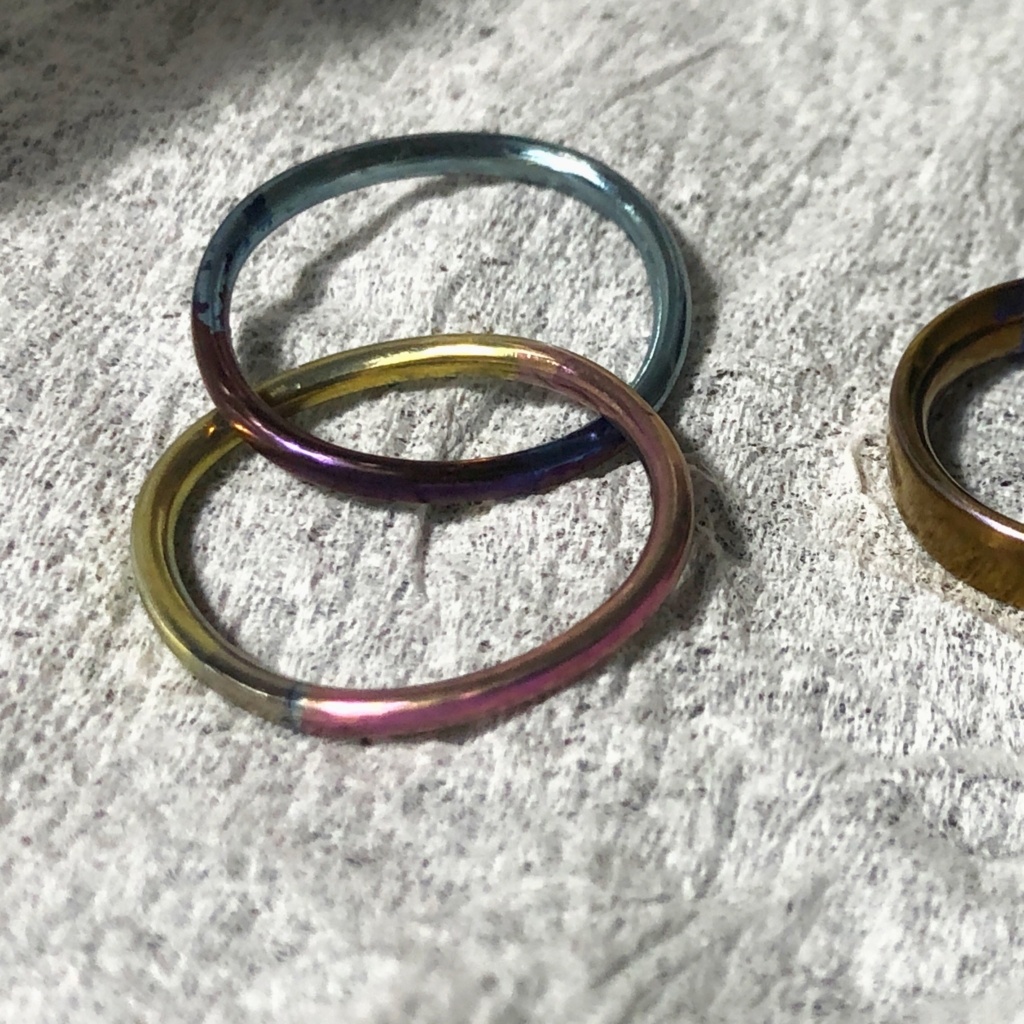
From here, it is titanium finished in a matte tone. From here, we are doing pretreatment such as etching.
I wrote that it was a grayish color in No.7 and 8, but since No.9 and later, pretreatment was performed firmly, so it did not gray even at the same voltage, and it was beautifully colored. Still, it became a little grayish at 110V.

This graying phenomenon seems to be related to the phenomenon of spark voltage.
When a voltage is applied to titanium, a spark occurs when a certain voltage is exceeded.
If the voltage is applied above the voltage from which this spark comes out, the color does not color beautifully and becomes a grayish color.
This spark voltage seems to be different depending on the solution, and the spark voltage seems to be higher in phosphoric acid and sulfuric acid.
Therefore, if you want to produce a color of high voltage such as green, it is better to use phosphoric acid.
The relationship between voltage and color tone in sulfuric acid is summarized in this article, so please refer to it if you like.
Effects of phosphoric acid concentration
The results introduced so far have been color tone at 10% phosphate concentration.
I was worried about what would happen if the concentration changed, so I examined it. The result is as follows:
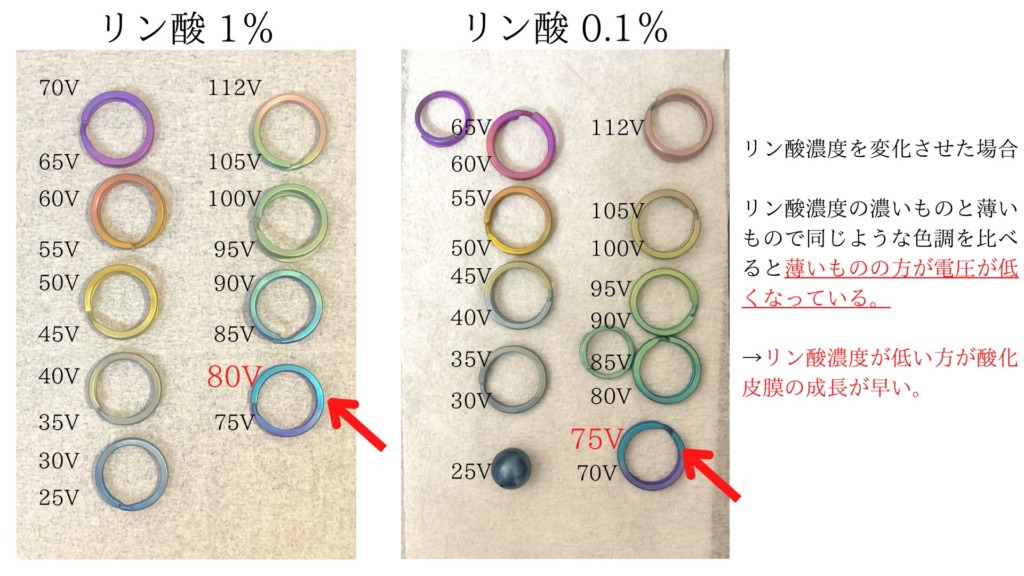

It was green at around 110V at a phosphate concentration of 10%, but when it was less than 1%, it became a pink color at almost the same voltage. (It’s like grayish pink.
Even if 1% and 0.1% are compared, lower concentrations lead to the same color tone at lower voltages.
In other words, I think that the thinner the concentration, the faster the oxide film grows.
Therefore, it seems better to reduce the concentration when you want green, which is a color of high voltage.
However, it seems not to say that it only has to be thinned to where.
When I tried to reduce it to 0.01%, I was able to get a similar color at the same voltage, but the color is quite sparse.
I think that the current probably does not flow well and the reaction is not stable, but it seems that it is useless to make it too thin.
It may be better to have a darker concentration when you want to control subtle colors that the higher the concentration, the slower the growth of the oxide film.
How to throw away the solution
It is better to store the solution that has been used in a separate bottle basically.
If you want to dispose of it, if it is a small amount without flushing it into the drain at home, it is discarded with garbage that burns by soaking in old cloth etc., and if it is a large amount, the city waterworks bureau responded that I would like you to ask the purchased supplier to dispose of it.
I would like to soak it in old cloth and dispose of it when it is necessary to dispose of it.
When rin flows into rivers, it becomes nutrients such as microorganisms and algae, and there is a risk of destroying ecosystems such as rivers, and its concentration is strictly controlled.
Don’t let it flow from your home drain just a little.
I think that it is certain to contact your waterworks bureau to be accurate.
Method for removing oxide film
If you experiment like this, you will soon lose the test piece.
In such a time, it is easy to redo it by dissolving the oxide film with a detergent that can be easily obtained.
Summary
How was it?
There was a paper that uses a mixture of sulfuric acid and phosphoric acid, so I would like to try it when mixing phosphoric acid and sulfuric acid, and I will summarize this in the article in the future.
In addition, I will summarize what kind of difference there is from sulfuric acid in the future.
As I wrote in No.7 and 8, when I tried using a titanium ring that did not have mirror gloss processing, it did not become a completely beautiful color, and it only turned to a gray color, so it seems better to basically use a mirror gloss.
We will introduce how to make a titanium ring mirror gloss in this article, so please take a look here as well.
We are thinking of experimenting with various conditions in the future, and we will update the above table every time, so I would be happy if you could visit it regularly.







Discussion
New Comments
No comments yet. Be the first one!